Class-11 Environmental Chemistry
General Introduction
The term ‘environment’ has gained a lot of importance during these years due to depleting resources and increasing population. Due the imbalance of the demand and the availability of natural resources, severalserious issues have come up that needs immediate attention for the survival of life on our planet earth!
The word environment embraces all living and non-living things existing on the earth and its atmosphere. It is the sum of all physical, chemical, biological, economic and social factors that interact to maintain equilibrium. Environmental studies focus mainly on human interaction with the environment. It is a broad field of study that includes disciplines such as physics, chemistry, biology, geology, economics and social sciences. For instance, the plants, animals and other human beings are part of a man’s environment. They constitute this biological environment. The lands on which we live and grows crops, the water that we drink and water we use for irrigation of crops, and the air that we breathes, are part of his physical environment. Apart from this, meteorological factors like sunlight, rains, temperature and wind also form a part of his physical environment. Each component of the environment is important for man’s well-being. Changes in the environment can take place by action of living beings. Man, through his acts, modifies the environment more than any other creature. Herein lays the danger to the environment. If man cannot act judiciously, and if his acts drastically change the environment, the consequences may recoil upon him and other creatures around him.
In this unit, the focus will be environmental chemistry which deals with study of the sources, reactions, transport, effects and fates of chemical species in the air, soil and water environments and the effect of human activity on these. With the rapidly increasing population of the Earth and continually advancingtechnology, human activities have an ever-increasing influence on thechemistry of the environment. Today we realize that our activities can have not only local and regionalbut also global consequences.
Environmental Pollution
Environmental pollution may be defined as the adverse effect on the environment produced either by a natural source or by human activity. Any substance, living or non-living, which causes pollution, is known as a pollutant. Introduction of pollutants into the environment causes instability, disorder or damage to the ecosystem or living organisms. A substance is considered as a pollutant if it is present in concentrations harmful to the natural environment. For example, phosphate and nitrate is added to soil to increase plant growth, but excessive concentration of these ions in water can be toxic.
A substance not present in nature, but introduced by human activity into the environment is called a contaminant. It causes deviation in the normal composition of the environment. A contaminant is classified as pollutant when it exceeds its limit (threshold) value and causes harmful effects.
Types of Pollutants
A pollutant can be solid, liquid or gaseous substance present in undesirable concentrations that is harmful to environment. On the basis of natural disposal, pollutants can be categorized as degradable and non-degradable. The degradable pollutants are those which can be readily broken down by natural processes. For instance, paper, domestic sewage, discarded vegetables, organic wastes etc, are degradable or non-persistent pollutants. Pollution due to such pollutants can be reduced by using appropriate methods.
On the other hand, non-degradable pollutants are those which cannot be readily broken down by natural processes, for example, plastics, DDT, nuclear waste, heavy metals such as lead, mercury etc. Pollution due to such pollutants is difficult to control as they persist in the environment in their unchanged form and keep on accumulating.
A pollutant originates from a source and enters the environment through air, water or soil. Human beings are directly dependent on these natural resources for their existence and basic needs of food, fuel and shelter. We need air to breathe, water to drink and soil to produce grains! So it is incumbent upon us to save our natural resources in order to save humanity form extinction. Industrial wastes, chemical fertilizers and pesticides have caused damage to the soil and affected the ground water. Excessive use of motor vehicles, deforestation and exhaust form industries has deteriorated the quality of air. It is therefore essential to find ways for restoring and conserving these natural resources.
Pollutants may be released to the environment by natural sources or by manmade sources. For example, emission of sulphur dioxide during a volcanic eruption is a natural source of this toxic gas.
Burning of fossil fuels is a man-made source of atmospheric sulphur dioxide.
The man-made sources of pollution are also called anthropogenic sources. The release of pollutants from anthropogenic sources is, in turn, attributed to excessive usage of energy resources, mainly fossil fuels, such as coal, petroleum and natural gas. The dependence on fossil fuels can be drastically
reduced by using alternative non-conventional energy resources. One such resource is the solar energy. It does not cause air pollution. Moreover, it is a renewable energy source as compared to fossil fuels which are fast depleting.
Conservation of Natural Resources
The natural resources on which the human survival depends are a component of biosphere. The latter is a thin shell of the earth including the oceans and the atmosphere, where the living organisms interact with non-living entities. The natural resources in the biosphere are broadly classified into three types: Air, water and land. Pollution despoils all these three components. The problems of air pollution and water pollution are discussed in Sections 14.3 and 14.4 respectively. The quality of land resources – also called terrestrial resources – in a particular area is directly or indirectly dependent on the quality of soil in that zone. Soil serves as a medium of entry of the nutrients into the living beings. The plants absorb the nutrients from the soil through their roots. With these they synthesize biomolecules upon which they, as well as other living organisms depend.
Soil is defined as a thin layer on the earth’s surface formed by the interaction of crustal rocks, sunlight, atmosphere and living organisms. Soil without living organisms is called mineral substrate. The term soil profile refers to a series of distinct horizontal layers of different compositions and characteristics. Each layer of soil is called a horizon. The uppermost layer of soil is called horizon A or topsoil. This layer is rich in inorganic nutrients and humus. Beneath the topsoil is the subsoil or horizon B. In addition to inorganic nutrients, this layer has a small proportion of organic matter. Beneath the subsoil is a layer of weathered rock material. It is designated as horizon C. The parent rock material occupies the lowest layer or horizon D. Since soil supports life, it must be conserved. The contaminants originating from industrial and agricultural processes, as byproducts or as waste materials, bring down the quality of soil. Appreciable quantities of heavy metals, such as lead and mercury have often been detected in soil samples in the vicinity of metallurgical and industrial units. Likewise the soil in fields and farmlands is invariably contaminated with pesticides like DDT and fertilizers like urea.
Yet another threat to soil arises due to the phenomena of erosion. Soil erosion refers to the weathering away of soil and its transportation by water or wind. Loss of vegetative cover and deforestation increase the rate of erosion. Erosion depletes the nutrients present in the soil, leading to fall in productivity. Various soil conservation techniques reduce the erosion rates by maintaining protective cover over the soil. For example, rotation of crop ensures that the land is covered with vegetation throughout the year. In hilly regions, the most practical method of reducing erosion is terrace farming. Leveled areas are dug out at right angle to the slope to retain water and prevent erosion.
Soil erosion caused by wind may be reduced by installing windbrakes. This practice involves planting trees that protect the soil from full force of the wind. Winbrakes reduce the speed of wind and hence reduce the quantity of soil that it can carry away.
Air Pollution
The earth’s atmosphere is the protective blanket of gases that surrounds the earth and protects the earth from the harsh environment of outer space. The composition of these gases varies with the altitude forming different regions or layers.(i) The lowest region of the atmosphere where life exists is known as troposphere. It extends to a height of 14 km from sea level and contains about 80% of the total air (mixture of N2 – 78%, O2 – 21%, Ar – 1%, CO2 – 0.03% and other trace gases) and almost all of water vapours. All the weather changes, turbulence and clouds formation occur in this layer. Temperature and water vapor content of the troposphere decrease rapidly with altitude.
(ii) The next layer within 14 to 50 km above sea level is known as stratosphere. It contains nitrogen, oxygen, ozone and traces of water vapour. Presence of ozone in the stratosphere protects us from harmful ultraviolet rays from the sun that causes skin cancer. There is very little air in this layer and no clouds. Stratosphere is thus not turbulent and that’s why jet airplanes prefer to fly in this layer.
(iii) Above stratosphere is the mesosphere where meteors and rock fragments burn up. Above that is the ionosphere or thermosphere where aurora takes place. The ionosphere is responsible for reflecting
radio waves making long-distance radio communication possible.
Atmospheric Pollution
The atmospheric pollution has caused considerable damaging effect in the troposphere as well as stratosphere. Let us study the effect of pollutants on these layers of atmosphere.Tropospheric Pollution
Environmental pollutants in the troposphere may be gaseous or particulate (suspended solid particles) in nature.
1. Gaseous air pollutants: Oxides of sulphur, carbon, nitrogen, H2S, hydrocarbons, O3 and other oxidants.
2. Particulate pollutants: Dust, fumes, smoke, ash, mist, smog etc.
These gaseous and particulate pollutants can further be divided into two main categories depending on their origin as primary and secondary pollutants. Primary pollutants are those which are directly emitted from the sources. Some examples are particulate matter such as ash, smoke, mist, dust, etc. and inorganic gases such as SO2, CO, NO2, H2S and radioactive substances. On the other hand, secondary pollutants are those which are formed in the atmosphere by chemical interactions among primary pollutants. Examples of a secondary pollutant are ozone, which is formed when hydrocarbons (HC) and nitrogen oxides (NOx) combine in the presence of sunlight; NO2, which is formed as NO combines with oxygen in the air; and acid rain, which is formed when sulfur dioxide or nitrogen oxides react with water. Some examples are SO3, NO2, O3, etc.
Major air pollutants can be divided into two categories. Inorganic gases and particulates.
Gaseous Air Pollutants
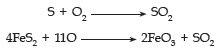
The majority of gaseous air pollutants are inducted into the atmosphere by combustion of fossil fuels as energy resources.(a) Oxides of sulphur: These are the most harmful of the common gaseous pollutants. The combustion of any sulphur containing fossil fuels produces SO2 and SO3 is the major constituent of the mixture (about 97%)
Smelting of sulphide ores and petroleum refining operations too increase the atmospheric load of sulphur oxides.
The oxides of sulphur affect respiratory tract producing nose, eye and lung irritation. SO2 is also considered to cause cough, shortness of breath and spasm of larynx. SO2 is also harmful for plants. It causes damage to leaf tissues and causes chlorosis (a bleaching or yellowing of normally green portions of the leaves). In areas having a high SO2 concentration, formation of H2SO4 causes damage to plants.
This causes deterioration of buildings, statues, etc. The problems arising due to sulphur oxides pollution may be mitigated by getting rid of sulphur content of fossil fuels.
(b) Oxides of nitrogen: The main constituents of dry air at sea level are: nitrogen 78% and oxygen (20%) by volume. In normal case, these two do not react but at high temperatures, they react to form nitric oxide. Thus burning of coal is a major source of nitrogen oxides in the environment. Emission from automobiles too adds nitrogen oxides to the air. Nitric oxide (NO) itself will have no adverse effect on human health. But when it is oxidised to NO2 with atmospheric oxygen or ozone, it is extremely toxic to living tissue and harmful to paints, textiles and metals.
NO2 is oxidised to NO3- ion and washed into the soil where it serves as a fertiliser. The most harmful effect of the oxides of nitrogen is in the production of photochemical smog.












No comments:
Post a Comment