Origin of Organic Chemistry as a Separate Branch of Chemistry
Early concepts of organic chemistry
Chemical compounds are divided into two main classes, inorganic and organic. Earlier, all substances were classified on the basis of the sources from which they were derived. Hence, all compounds that were of mineral origin were known as inorganic compounds (e.g. table salt, rock salt, marble, etc.) and all compounds of vegetable and animal origin were known as organic compounds (e.g. alcohol, citric acid, oxalic acid, esters, etc.). Prior to 1828, all organic compounds had been obtained from organisms or their remains. The belief then was that the synthesis of organic compounds from inorganic compounds in the laboratory was impossible. All efforts had failed, and scientists became con¬vinced that some ‘vital force’ that living organisms had, was necessary to make an organic compound. The name 'organic', thus, originated with early chemists, who believed that organic compounds could be formed only through the action of a vital force found in living organisms. So, no effort was made to produce organic compounds in the laboratory for many decades. This was known as vital force theory.
Modern concepts of organic chemistry
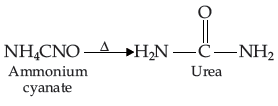
The essential distinction between organic and inorganic substances was revolutionized by Friedrich Wohler’s accidental synthesis of urea from inorganic substances in 1828. Never before had an organic compound been synthesized from inorganic substance!
Friedrich Wöhler
Source: chemistryexplained.com
Wöhler with his co-worker Justus Liebig
published an investigation related to ‘oil of
bitter almonds’. Through their experiments they
proved that a group of carbon, hydrogen and
oxygen atoms can behave like an element, take
the place of an element, and can be exchanged
for elements in chemical compounds. This lead
to the foundation of the doctrine of compound
radicals.
In the years that followed, many other different organic compounds were synthesised by different scientists in laboratories. This gave the basis for rejection of the vital force theory.
It had become evident, however, that most of the organic compounds formed by living cells contained carbon. So, the emphasis was shifted from origin to composition. Organic chemistry, thus, is now known as the branch of chemistry dealing with the study of carbon compounds.
Organic chemistry is a discipline of chemistry that deals primarily with properties, composition, synthesis of carbon compounds and their derivatives.
Organic chemistry is studied as a separate discipline because of the following reasons:
1. Carbon being a versatile element can form a large number of compounds.
2. Carbon has a unique character, which includes tetra-covalency, catenation, strong C-C bonding and the tendency to form multiple bonding (i.e. single, double, and triple bonds through covalent bond¬ing with hydrogen and other atoms).
3. Thus a large number of organic compounds are known that are classified into different families. Each family consists of compounds that have a chemically active centre called the functional group.
4. These compounds have unique chemical and physical properties. The bonding and structural features of a compound are manifested in its physical properties (melting point, boiling point, solubility, etc.) which in turn depends on the nature of atoms constituting its structural units and nature of forces holding its units together. In covalent compounds, intermolecular forces hold these molecules together. The chemical properties, on the other hand, are influenced by electronic displacements in the molecule.
Importance of organic chemistry
Organic chemistry is related to our daily life activities. We are all made of mainly organic compounds! The food we eat (carbohydrates, proteins, fats, etc.), the clothes we wear (cotton, silk, wool, nylon, rayon, dacron, etc.), our shelter (wood, paints, varnishes, etc.), the fuels we consume (natural gas, petroleum products, coal, etc.), medicines and drugs (penicillin G, streptomycin, etc.), insecticides and pesticides, biomolecules (hormones, steroids, vitamins and enzymes, etc.), antiseptics and anaesthetics, pigments and dyes, paper and ink, photographic films and developers, perfumes and flavours, plastics, rubber, resins, propellants, explosives, soaps, detergents, refrigerants, etc. are all made up of organic compounds.
Can you imagine your life without carbon compounds i.e. organic compounds? Do you know why carbon forms such a large number of compounds? Can you guess why diamond and graphite are so different, though they are both made up of carbon? The solution to all these and many more questions lies in Organic Chemistry, which is the study of these organic compounds.
In this unit, we shall study some basic concepts of carbon compounds like their structure and bonding, functional groups, classifications, nomenclature, structural and stereoisomerism, fission (or cleavage) of bonds, types of reagents and types of organic reactions, aromaticity, acidity and basicity. You must try to understand all these concepts as they are the basis for understanding all the other topics in organic chemistry.
Structure of Organic Compounds
The properties of organic compounds can be best explained with the help of structure of these compounds. So it becomes very essential for us to know the structure of organic compounds which can in turn be explained with the help of the concept of hybridization. You have already studied hybridization in the chemical bonding unit.
Here we shall study the three different types of hybridizations exhibited by carbon in different organic compounds, viz. sp3, sp2 and sp. You may be surprised to know that different carbon atoms may be present in different hybridizations in the same organic compound. We shall try to understand the different hybridizations of carbon with the help of some simple examples and then extend the concept in order to find the hybridization of any carbon in any given organic compound.

No comments:
Post a Comment