Types of hybridization in carbon compounds
We shall now try to understand the different hybridizations of carbon in organic compounds. Carbon in organic compounds exhibits three types of hybridization. Namely, sp3, sp2 and sp.
Effect of hybridization on organic compounds:
• Bond length
• Bond energy
Concept of Hybridization
sp3 hybridization
Let us try to understand the sp3 hybridization with the help of structure of methane (CH4). The electronic configuration of a carbon atom in its lowest energy stateº, called the ground state is represented as (I) where there are only two unpaired electrons. But for the formation of a molecule like CH4, 4 unpaired electrons are required. Thus, the carbon atom gets excited to a higher energy state (one electron from 2s is excited to 2p) called the excited state (II). (Figure: 6.1)
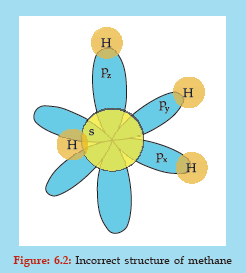
The carbon atom now has the required four electrons and must combine with four hydrogen atoms to form a methane molecule. But it does not! Because, if this happens, then, as we know, the s orbital is spherical and the p orbital is dumb-bell shaped, the methane molecule must have the structure as shown below (Figure: 6.2) where three C-H bonds are longer than one C-H bond and the some angles between C-H bonds are 90° (due to 90° relative orientation of px, py and pz).
But this is not how the real molecule is! In fact, methane has a tetrahedral structure with all the C-H bonds are of equal length and all the bond angles 109°28′ as shown by crystallographic, X-ray and other studies. This can be accounted by the concept of hybridization. In hybridization, equal number of hybrid orbitals result from reorganisation of atomic orbitals. Here, the four atomic orbitals, one s orbital (2s) and three p orbitals (2px , 2py , and 2pz ) undergo hybridization and give rise to an equal
number of (i.e., four sp3) hybrid orbitals.
These are called sp3 hybrid orbitals as they have one part the character of an s orbital and three parts the character of a p orbital. They arrange themselves, with the maximum separation from each other, in a tetrahedral manner and 109°28' as the angle of orientation with respect to each other as shown in (Figure 6.3).
These four sp3 hybrid orbitals now overlap with the s orbitals of four hydrogen atoms (one each) to give a tetrahedral structure for methane having all equivalent C-H sigma bonds and all bond angles as 109°28' or 109.5° (Fig.6.4).
Example: Ammonia molecule
sp2 hybridization
Let us try to understand the sp2 hybridization with the help of structure of ethene (C2H4). In ethene, the carbon-carbon double bond has one s (sigma) bond and one π (pi) bond. Each carbon is further attached to two hydrogen atoms by s bonds as shown below
As discussed in the earlier case, the excited states of each carbon can be shown as in Figure: 6.5:
As you already know that the π bond is always formed by the overlap of unhybridised p orbitals (as the [p bond is a two way overlap, one above and one below the inter-nuclear axis and thus not possible with the hybrid orbitals where electron density in one lobe is very small and cannot take part in the bonding via sufficient overlap). Let us assume Z to be the internuclear axis. Thus, one of the 2py or 2px orbitals of one carbon atom overlaps sideways with 2py or 2px orbitals of the other carbon atom (here, let us say 2px) respectively to form the p bond as shown in Figure: 6.6.
Now, in the valence shell, each carbon atom is left with one s orbital (2s) and two p orbitals (2pz and 2py), which undergo hybridization and give rise to three sp2 hybrid orbitals.
These are called sp2 hybrid orbitals to indicate that they have one part the character of s orbital and two parts the character of a p orbital. These three sp2 orbitals are directed towards the corners of a regular triangle with angles of 120° between them. The unhybridised p (2px) orbital is perpendicular to the plane of the triangle formed by the sp2 hybrid orbitals. This gives the structure of ethene as shown in Figure: 6.7.
There are a total of five s bonds in the ethene molecule. One carbon-carbon s bond formed via the overlap of one sp2 hybrid orbital of each carbon. Four carbon-hydrogen s bonds formed by the remaining two-sp2 hybrid orbitals on each carbon overlapping with two hydrogen atoms (one each), i.e. two σ bonds on each carbon atoms. The bond angle between each bond is 120°. All the six atoms (two carbon and four hydrogen) lie in the same plane and the π orbitals are perpendicular to this plane. Ethene has thus a planar structure.
sp hybridization
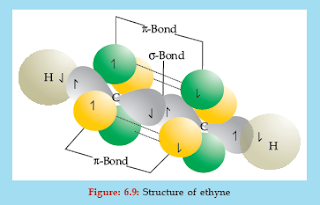
Let us try to understand the sp hybridization with the help of structure of ethyne (C2H2). In ethyne, the carbon-carbon triple bond has one σ (sigma) bond and two π (pi) bonds as shown below
As earlier, the excited states of each carbon atom can be shown as follows:
Let us assume Z to be the internuclear axis, then the 2py and 2px of one-carbon overlap sideways with a 2py and 2px of the other carbon atom respectively to form two π bonds. Each carbon atom is left with one s orbital (2s) and one p orbital (2pz), which undergo hybridization and give rise to two sp hybrid orbitals.
These hybrid orbitals are called sp orbitals to indicate that they have one part the character of the s orbital and one part the character of the p orbital. These two sp hybrid orbitals are directed in a linear fashion with angles of 180° between them (Figure: 6.9).
One sp hybrid orbital of each carbon overlaps to form a carbon-carbon s bond. The remaining sp hybrid orbital on each carbon overlaps with one hydrogen atom each to give two s bonds. Thus a total of three s bonds are found in the ethyne molecule. The bond angle between each bond is 180°. Acetylene is a linear molecule as all the four atoms (two carbon and two hydrogen) lie along the same straight line. Also, it has a cylindrical symmetry about the inter-nuclear axis due to the p electron cloud present in a cylindrical manner.














No comments:
Post a Comment